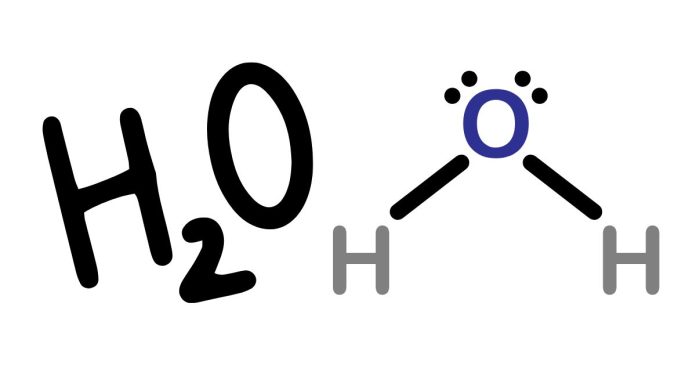
If two students create Lewis dot diagrams of H₂O, their diagrams should ideally reflect the correct structure of the molecule, but variations can arise depending on their understanding. Here’s a breakdown:
Correct Lewis Dot Structure of H₂O
- Valence Electrons: Oxygen has 6 valence electrons, and each hydrogen has 1, giving a total of 6+1+1=86 + 1 + 1 = 8 valence electrons for H₂O.
- Bonding:
- Oxygen forms two single bonds with hydrogen atoms.
- Oxygen has two lone pairs of electrons.
The correct Lewis structure looks like this:
..
H - O - H
..
Possible Scenarios for the Students’ Diagrams
- Correct Diagram:
If both students drew the above structure, they correctly accounted for the 8 valence electrons, bonding pairs, and lone pairs. - Common Mistakes:
- Student 1: They may have missed the lone pairs on oxygen or added extra bonds, such as double bonds between O and H.
- Student 2: They might have misrepresented the electron distribution, for example, placing lone pairs on hydrogen or not completing oxygen’s octet.
Comparing the Two Diagrams
- Check the total number of electrons to ensure all 8 are represented.
- Verify that oxygen forms two single bonds and has two lone pairs.
- Ensure hydrogen has only two electrons (a complete duplet).
Discussion
If the students’ diagrams differ, it provides an opportunity to clarify:
- Octet Rule: Why oxygen needs a complete octet.
- Hydrogen’s Duplet: Why hydrogen only forms single bonds.
- Electron Distribution: The importance of accurately placing lone pairs.
Encouraging both students to cross-check their work against these principles can help ensure they understand how to construct correct Lewis dot diagrams in the future.