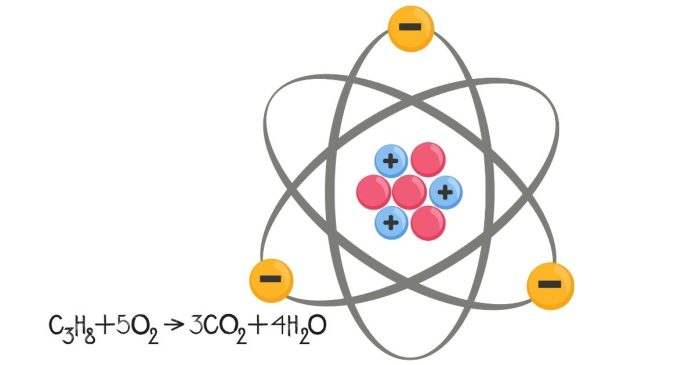
Here’s a breakdown of the symbols for protons, neutrons, and electrons in chemistry, including their characteristics and roles:
1. Protons (p or p⁺):
- Symbol: pp or p+p^+
- Charge: Positive (+1+1)
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Found in the nucleus of an atom.
- Role: Protons determine the atomic number of an element. This number is crucial because it defines the identity of an element. For example, an atom with 1 proton is hydrogen, with 6 protons is carbon, and so on. The number of protons in the nucleus also contributes to the positive charge of the nucleus.
2. Neutrons (n):
- Symbol: nn
- Charge: Neutral (no charge, 0)
- Mass: Approximately 1 atomic mass unit (amu), slightly more than a proton.
- Location: Found in the nucleus of an atom.
- Role: Neutrons help to stabilize the nucleus. They act as a buffer between protons, reducing the repulsive electrostatic forces between positively charged protons. The number of neutrons in an atom can vary (resulting in isotopes of an element), but the number of neutrons typically closely matches the number of protons in a stable atom.
3. Electrons (e⁻):
- Symbol: e−e^-
- Charge: Negative (−1-1)
- Mass: Negligible compared to protons and neutrons (about 9.11 × 1 0 − 31 9.11×10 −31 kg or 1 / 1836 1/1836 of a proton).
- Location: Orbit around the nucleus in electron shells or energy levels.
- Role: Electrons are involved in chemical reactions and bonding. The distribution of electrons in an atom’s electron shells determines how atoms interact with each other. The number of electrons in a neutral atom equals the number of protons, ensuring the atom as a whole has no charge. However, electrons can be gained or lost, which results in ions (charged atoms).
Summary of Charges:
- Proton: Positive charge (+1)
- Neutron: No charge (0)
- Electron: Negative charge (-1)
Atomic Structure Overview:
- Nucleus: Contains protons and neutrons.
- Electron cloud: Electrons move around the nucleus in various energy levels or orbitals.
These subatomic particles are the fundamental components of atoms, and their interactions define the chemical behavior of matter.