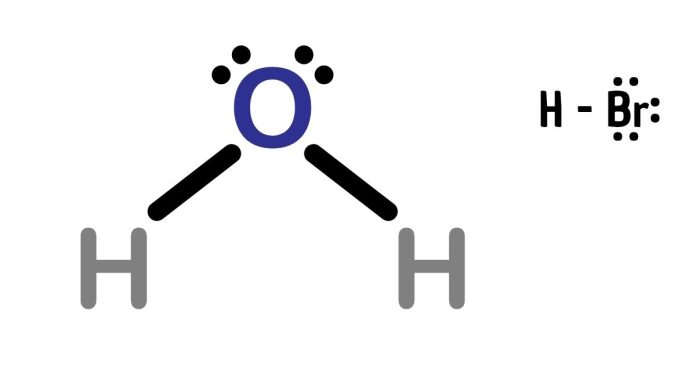
The Lewis dot structure of hydrogen bromide (HBr) is relatively simple. Here’s how to draw it:
- Identify the atoms involved:
- H (hydrogen) has 1 valence electron.
- Br (bromine) has 7 valence electrons.
- Arrange the atoms:
- Hydrogen (H) is placed on the left, and bromine (Br) on the right. Since H is only capable of forming one bond, it will bond with Br.
- Draw the bond:
- A single bond (a pair of electrons) connects H and Br.
- Complete the octet for bromine:
- Bromine has 7 valence electrons, and after forming a bond with hydrogen, it has 6 electrons remaining. These are placed as 3 lone pairs around the bromine atom.
The final structure looks like this:
H : Br
Where:
- The single bond (represented by a colon) between H and Br shows the shared pair of electrons.
- Bromine has three lone pairs (represented by dots) around it to complete its octet.
This is the Lewis dot structure of HBr.