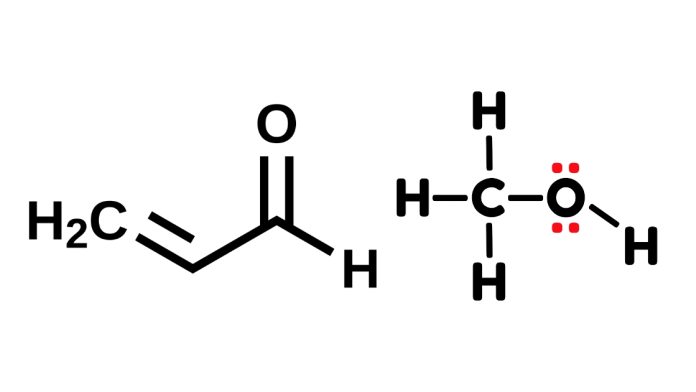
Draw the most stable Lewis structure of acrolein.
- Carbon Backbone: The structure has three carbon atoms in a chain.
- Double Bonds:
- One double bond between the first carbon and oxygen atom (C=O).
- Another double bond between the second and third carbon atoms (C=C).
- Hydrogen Atoms:
- Two hydrogens are attached to the third carbon atom.
- One hydrogen is attached to the first carbon atom.
- One hydrogen is attached to the second carbon atom.
Steps to Draw the Structure:
- Place the Atoms: Arrange the three carbon atoms in a chain.
- Add Bonds:
- Connect the first carbon to oxygen with a double bond (C=O).
- Connect the second carbon to the third carbon with a double bond (C=C).
- Attach the remaining single bonds to the appropriate hydrogen atoms.
- Complete the Octet Rule:
- Ensure each atom (except hydrogen) has a complete octet of electrons.
- Formal Charges:
- The structure should minimize formal charges on all atoms, resulting in the most stable configuration.
The final structure looks like this:
H H
\ /
H—C = C—C=O
|
H