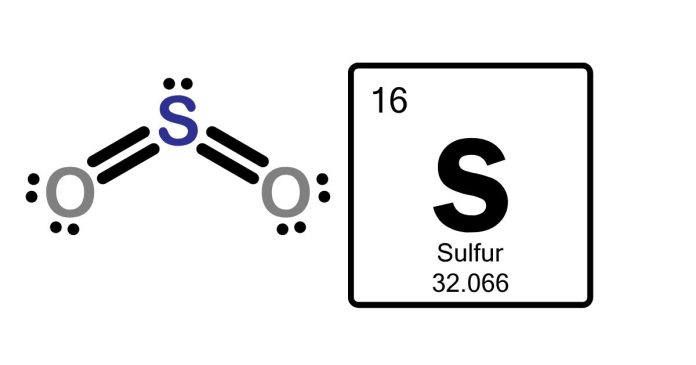
The central idea is about the Lewis structure of the S3 molecule supporting details include:
- forms a cyclic (triangular) shape.
- Each sulfur atom has two lone pairs and is bonded to two others via single bonds.
- The total valence electrons (18) satisfy the octet rule for all sulfur atoms.
Therefore, details not directly explaining or relevant to this structure do not support the central idea.
Examples:
Non-Supporting Details:
- “Sulfur is commonly found in volcanic emissions and is used to make sulfuric acid.” (This is unrelated to the molecule’s structure.)
- “Sulfur atoms can form S8. rings in their elemental state.” (This is about another form of sulfur, not S3)
- “Sulfur compounds often have a strong smell, like hydrogen sulfide gas.” (Irrelevant to drawing the Lewis structure.)