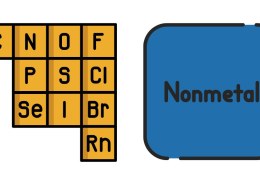
The most active nonmetals are located on the right side of the periodic table, specifically in the halogen group (Group 17 or Group VIIA). These elements include:
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
- Astatine (At)
Why Are Halogens the Most Active Nonmetals?
- High Electronegativity: Halogens have a strong tendency to attract electrons to complete their outer electron shells.
- High Reactivity: They are one electron short of a full valence shell, making them highly reactive with metals and other substances.
- Decreasing Reactivity Down the Group: Fluorine is the most reactive nonmetal, followed by chlorine, bromine, and so on. Reactivity decreases as you move down the group due to increasing atomic size and decreasing electronegativity.
In summary, the most active nonmetals are found in Group 17, with fluorine being the most reactive of all. These elements play crucial roles in chemical reactions, from forming salts to being used in disinfectants and industrial processes.