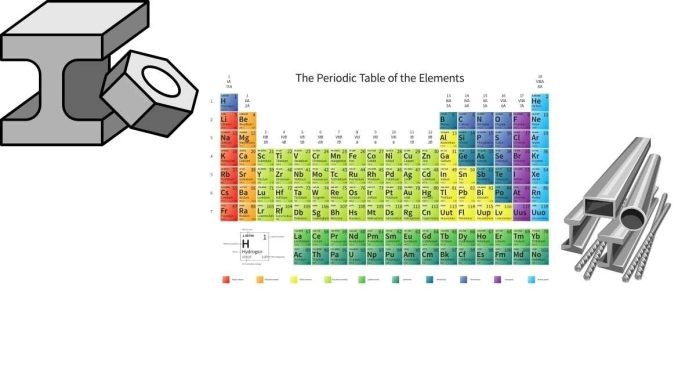
Most nonmetals in the periodic table are located in the upper right section, with a few exceptions. Here’s a general breakdown:
- Group 18 (Noble Gases): The noble gases (e.g., helium, neon, argon, krypton, xenon, radon) are all nonmetals and are found in the far-right column of the periodic table.
- Groups 15-17: These groups contain other nonmetals:
- Group 15: Nitrogen (N), phosphorus (P), arsenic (As) (the first two are nonmetals, while arsenic is a metalloid).
- Group 16: Oxygen (O), sulfur (S), selenium (Se), tellurium (Te) (the first two are nonmetals, while tellurium is a metalloid).
- Group 17: The halogens, which are nonmetals, include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
- Other nonmetals: In addition to these groups, some nonmetals appear in other parts of the table, such as hydrogen (H), which is in Group 1 but is a nonmetal.
Summary:
- Most nonmetals are in Groups 14-18.
- Nonmetals are predominantly located in the upper right of the table, with the exception of hydrogen, which is placed at the top of Group 1.