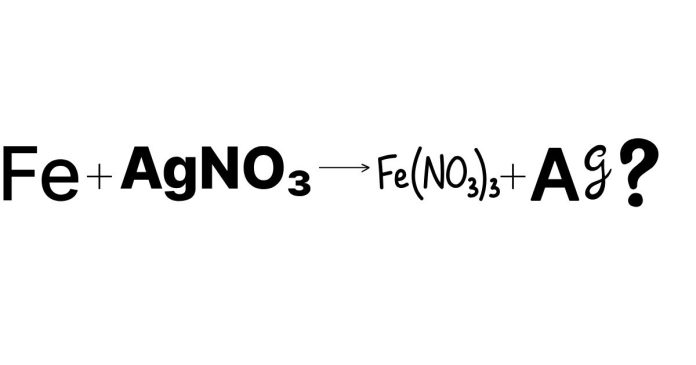In many chemical reactions, one substance undergoes reduction (gaining electrons), while another undergoes oxidation (losing electrons). The substance that donates electrons to another is called the reducing agent. In the reaction:
Fe + AgNO₃ → Fe(NO₃)₂ + Ag
We are dealing with a redox (reduction-oxidation) reaction, and understanding which substance is the reducing agent helps to clarify the flow of electrons. Let’s break down the reaction and identify the roles of the involved substances.
Understanding the Reaction
The given reaction involves two substances:
- Iron (Fe), a solid metal, reacts with silver nitrate (AgNO₃) in aqueous form.
- Iron nitrate (Fe(NO₃)₂) and silver (Ag) are produced as products.
This is a single displacement reaction, where iron displaces silver from silver nitrate, resulting in the formation of solid silver and iron(II) nitrate.
Oxidation and Reduction
To identify the reducing agent, we need to determine which elements undergo oxidation and which undergo reduction:
- Oxidation (Iron):
- Iron starts as a neutral metal (Fe, oxidation state = 0) and reacts to form iron(II) nitrate (Fe(NO₃)₂). In this process, iron loses two electrons to become iron(II) (Fe²⁺), which means iron is oxidized.
- Oxidation: Fe → Fe²⁺ + 2e⁻
- Reduction (Silver):
- Silver starts as a silver ion (Ag⁺, oxidation state +1) in the silver nitrate. It gains the electrons that iron loses, which causes silver ions to reduce to solid silver (Ag, oxidation state 0).
- Reduction: Ag⁺ + e⁻ → Ag
What Is the Reducing Agent?
The reducing agent is the substance that donates electrons to another substance during the reaction. Since iron (Fe) loses electrons (it undergoes oxidation), it is the reducing agent in this reaction. By losing electrons, iron facilitates the reduction of silver ions to solid silver.
Summary of the Reaction
- Iron (Fe) is the reducing agent because it donates electrons to reduce silver ions.
- Silver (Ag) is the substance being reduced, as it gains electrons to form solid silver.
- Iron (Fe) undergoes oxidation, and silver ions (Ag⁺) undergo reduction.
In the reaction Fe + AgNO₃ → Fe(NO₃)₂ + Ag, the reducing agent is iron (Fe). Iron donates electrons to reduce silver ions (Ag⁺) to metallic silver (Ag), while iron itself is oxidized to iron(II) ions (Fe²⁺). This highlights the key role of reducing agents in redox reactions: they facilitate the reduction of other substances by losing electrons themselves.