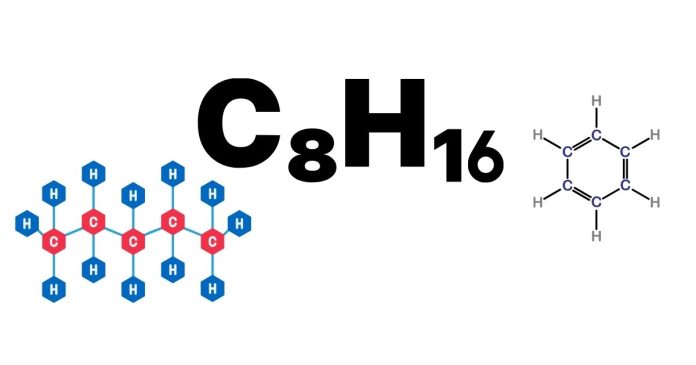
The chemical formula C₈H₁₆ represents a hydrocarbon composed of eight carbon (C) atoms and sixteen hydrogen (H) atoms. This molecular formula corresponds to a class of compounds called alkanes, which are saturated hydrocarbons. In the case of C₈H₁₆, the specific compound is known as octane.
Understanding Octane
The general formula for alkanes is CₙH₂ₙ₊₂, where “n” represents the number of carbon atoms. For octane, n = 8, and hence the formula is C₈H₁₆. Octane is a member of the alkane series, which means that it consists entirely of single bonds between carbon atoms and is saturated with hydrogen atoms.
Isomers of Octane
While octane is commonly used to refer to n-octane (a straight-chain alkane), there are also several isomers of octane, each with a slightly different structure. These isomers have the same molecular formula (C₈H₁₆) but differ in the arrangement of atoms. Some common isomers include:
- n-Octane: The straight-chain version of octane, where all the carbon atoms are connected in a single unbranched chain.
- Isooctane (2,2,4-Trimethylpentane): One of the most famous isomers, especially known for its high octane rating used in fuels.
- Other branched isomers: These include various arrangements of carbon chains, such as 2-methylheptane, 3-methylheptane, and more.
Importance of Octane
Octane is a crucial component in the fuel industry. It is a primary ingredient in gasoline, where its performance is evaluated by its octane rating. A higher octane rating indicates better resistance to engine knocking, which can cause engine damage.
- n-Octane has a lower octane rating compared to iso-octane, the reference compound used to measure the octane number in fuels.
- Isooctane, with a high octane rating, is often used as a benchmark in determining the quality of gasoline.
The hydrocarbon with the molecular formula C₈H₁₆ is named octane. This compound is significant not only as a part of the alkane family but also for its application in the fuel industry, where its different isomers, especially iso-octane, are used to determine fuel efficiency and engine performance. Whether straight-chain or branched, octane plays a fundamental role in the chemistry of combustion and energy production.