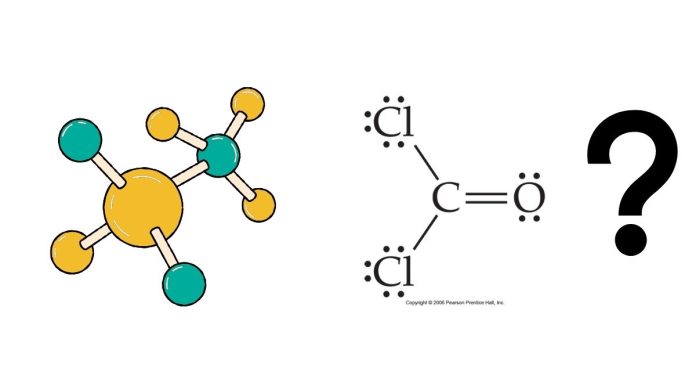
Understanding the molecular shape of a compound is crucial in predicting its chemical behavior, physical properties, and reactivity. One molecule that often comes up in discussions of molecular geometry is Cl₂CO, also known as phosgene. Let’s look at the molecular structure of Cl₂CO, including its geometry, bond angles, and why it takes on the shape it does.

Molecular Formula and Structure
The molecular formula of Cl₂CO represents one carbon atom (C), one oxygen atom (O), and two chlorine atoms (Cl). The carbon atom is at the center, double-bonded to oxygen and single-bonded to each chlorine atom.
Refer to the image above for a visual representation of the molecule’s structure. Notice how the bonds and angles form a distinct pattern.
Electron Domain and Molecular Geometry
Using VSEPR theory (Valence Shell Electron Pair Repulsion theory):
- Electron Domains: There are three regions of electron density around the central carbon atom—two single bonds with chlorine and one double bond with oxygen.
- Molecular Geometry: These regions arrange themselves as far apart as possible, resulting in a trigonal planar shape.
This geometry means all atoms lie in a single plane with bond angles approximately 120°. The image illustrates this planar geometry and the relative positions of the atoms.
Bond Angles and Symmetry
The molecular geometry of Cl₂CO results in bond angles of nearly 120°.
- The Cl-C-Cl and Cl-C=O angles are slightly adjusted by the electronegativity differences between the atoms, but the overall shape remains planar.
Polarity of Cl₂CO
Despite its symmetrical planar structure, Cl₂CO is a polar molecule.
- The C=O bond introduces significant polarity due to the high electronegativity of oxygen.
- The Cl-C bonds are also polar, but the molecule’s shape results in a net dipole moment, making it polar overall.
Applications and Safety
Cl₂CO has several industrial applications but is also highly toxic:
- Uses: In the manufacture of polycarbonate plastics and pesticides.
- Hazards: Phosgene is dangerous if inhaled and was historically used as a chemical weapon.
The molecular shape of Cl₂CO is trigonal planar, with bond angles of approximately 120°. The geometry arises from the sp² hybridization of the central carbon atom and the arrangement of bonding pairs. Understanding this structure is essential for appreciating the molecule’s properties and its applications in various fields.
The accompanying illustration provides a clear depiction of the molecule’s shape and highlights its chemical symmetry and planar geometry.