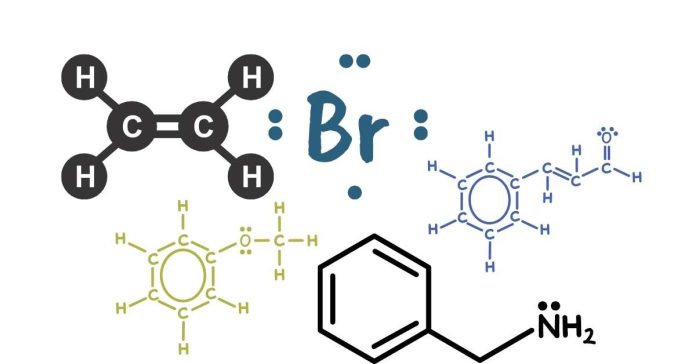
The Lewis structure of C2H2Br2 (1,2-dibromoethene) consists of two carbon atoms (C), two hydrogen atoms (H), and two bromine atoms (Br). Each carbon forms a single bond with one hydrogen and a double bond with the other carbon. The two bromine atoms are bonded to each carbon.
Here’s a simplified representation:
H – C = C – Br
|
Br
Each carbon atom follows the octet rule by forming bonds with hydrogen and bromine. The bromine atoms have three lone pairs of electrons. This structure reflects the bonding arrangement in the molecule.