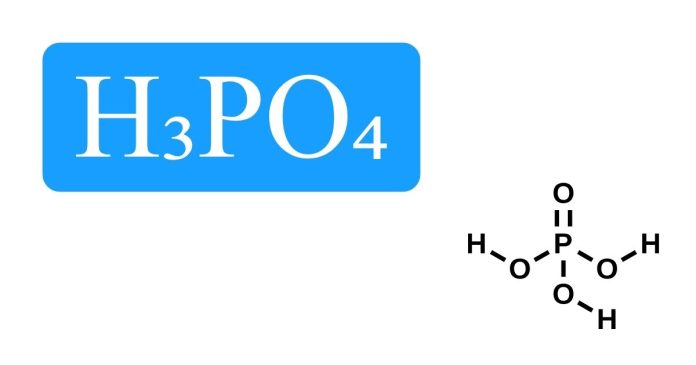
Phosphoric acid, scientifically known as H₃PO₄, is a widely used chemical compound found in a variety of industries, from food production to agriculture. However, you may have encountered the formula “2H₃PO₄” in certain contexts, leading to some confusion. So, what exactly does 2H₃PO₄ refer to?
Understanding the Formula
The chemical formula 2H₃PO₄ represents two molecules of phosphoric acid (H₃PO₄). Phosphoric acid itself consists of three hydrogen atoms (H), one phosphorus atom (P), and four oxygen atoms (O). The “2” before H₃PO₄ means that two separate molecules of phosphoric acid are involved in a given context, which could be relevant in various chemical reactions, industrial processes, or as a part of a mixture.
Phosphoric Acid (H₃PO₄) Overview
Phosphoric acid is a mineral acid, and it appears as a colorless, odorless liquid or solid at room temperature. It’s an important chemical in industries such as:
- Fertilizer Production: Phosphoric acid is a key ingredient in manufacturing fertilizers, especially those used to promote plant growth, such as superphosphate.
- Food Additives: In the food industry, phosphoric acid is used to acidify food and beverages like soft drinks. It imparts a tangy taste and helps preserve the product.
- Water Treatment: It can also be used in water treatment processes to adjust pH levels or remove impurities.
- Cleaning Products: Phosphoric acid is used in cleaning agents, especially those intended for removing rust and mineral stains.
The Role of 2H₃PO₄ in Chemical Reactions
When referring to 2H₃PO₄, it may appear in chemical equations or formulations where two molecules of phosphoric acid are reacting or participating in a process. For example, in reactions where multiple moles of phosphoric acid are involved to produce a certain outcome, such as in the synthesis of phosphates or other phosphorus-containing compounds, 2H₃PO₄ would be indicated to show the amount of phosphoric acid being used.
Is There Any Difference Between H₃PO₄ and 2H₃PO₄?
There is no intrinsic difference in the basic properties of H₃PO₄ and 2H₃PO₄. The only difference is the quantity of phosphoric acid molecules involved. H₃PO₄ represents a single molecule of phosphoric acid, while 2H₃PO₄ refers to two molecules. This is important in stoichiometry, chemical reactions, and industrial processes where precise amounts of chemicals are needed.
Safety Considerations
Phosphoric acid, even in its pure form, is generally considered safe for most uses, though it should still be handled with care. It can cause skin irritation and should be kept away from eyes. In concentrated forms, it can be highly corrosive. As with any chemical, appropriate safety protocols should be followed.
While 2H₃PO₄ may seem like an unusual formula at first, it is simply an indication of two molecules of phosphoric acid. Phosphoric acid is a versatile and crucial compound in many industries, and understanding its uses and forms can help better appreciate its role in everyday products and processes. Whether in fertilizers, food, or industrial applications, phosphoric acid plays a key role in modern chemistry.