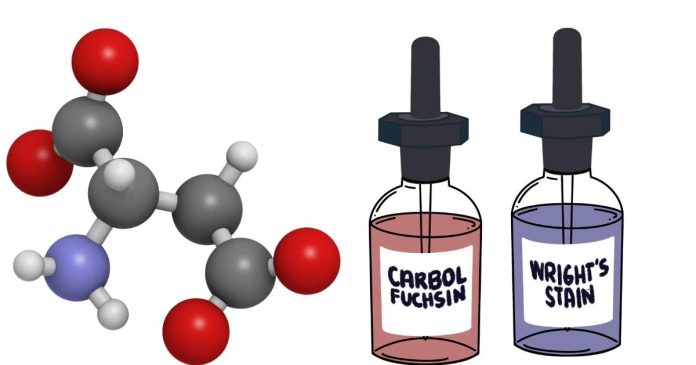
A carboxylic acid contains a functional group known as the carboxyl group. This group has the general structure:
-COOH
Now, let’s look at the components of the carboxyl group:
- Carbonyl group (C=O):
The carbonyl group consists of a carbon double-bonded to an oxygen atom (C=O). This part of the carboxyl group is polar, and the oxygen is electron-rich, making the carbonyl carbon somewhat electrophilic (i.e., susceptible to nucleophilic attack). - Hydroxyl group (OH):
Attached to the carbonyl carbon is a hydroxyl group (-OH), which is a hydrogen atom bonded to an oxygen atom. The oxygen in the hydroxyl group is electronegative, contributing to the polarity of the carboxyl group and allowing the molecule to participate in hydrogen bonding.
Together, these components form the carboxyl group (-COOH). The carboxyl group is what gives carboxylic acids their characteristic properties, including their ability to donate a proton (H⁺) from the hydroxyl group. This proton donation is what makes carboxylic acids acidic.
Additional details:
- Acidity: The carboxyl group makes carboxylic acids acidic because the hydrogen ion (H⁺) can be easily lost from the hydroxyl part of the group, forming a carboxylate anion (-COO⁻). This deprotonation is facilitated by the resonance stabilization of the conjugate base (the carboxylate anion), where the negative charge is delocalized between the two oxygen atoms.
- Resonance: The negative charge on the conjugate base (carboxylate ion) is delocalized between the two oxygen atoms, which stabilizes the anion and makes the proton more easily dissociable, enhancing the acidity of the molecule.
In terms of structure, carboxylic acids can be written as R-COOH, where R is any alkyl or aryl group. For example:
- Acetic acid (CH₃COOH): In acetic acid, the carboxyl group is attached to a methyl group (CH₃).
The carboxyl group can also participate in reactions such as esterification (to form esters), reduction (to form alcohols), or condensation reactions to form anhydrides.