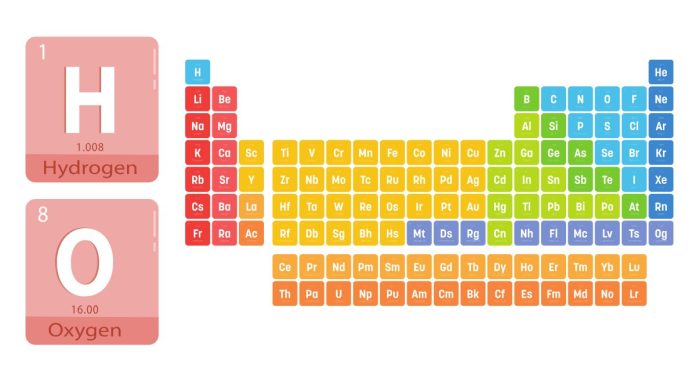
Elements in the same period of the periodic table share several important characteristics due to their similar electron configurations. Here’s a look at what elements in the same period have in common:
- Same Number of Electron Shells: Elements in the same period have the same number of electron shells or energy levels. For example, elements in period 2, such as lithium (Li) and neon (Ne), all have two electron shells.
- Gradual Change in Properties: As you move across a period from left to right, the properties of elements change gradually. This shift is seen in various characteristics, such as metallic to non-metallic properties. For instance, metals are found on the left, while non-metals are on the right.
- Increasing Atomic Number: The atomic number, which indicates the number of protons in the nucleus of an atom, increases as you move from left to right across a period. Each successive element has one more proton and one more electron than the previous one.
- Valence Electrons: While the number of electron shells remains the same, the number of valence electrons increases as you move across a period. This change significantly influences the chemical reactivity of the elements.
- Trends in Reactivity and Properties: As a result of these electron configuration changes, there are notable trends in reactivity, ionization energy, and electronegativity. For example, ionization energy and electronegativity tend to increase as you move across a period.
Overall, elements in the same period are connected by their electron shell structure and exhibit trends in chemical and physical properties. These trends help scientists predict how elements might behave in reactions and compounds.