Chemical reactions provide a fascinating insight into how substances interact and transform. One such reaction is the interaction between magnesium chloride (“MgCl₂”) and potassium hydroxide (“KOH”). This article explores the stoichiometry and chemistry behind this reaction, including calculations based on specific amounts of reactants.
The Reaction:
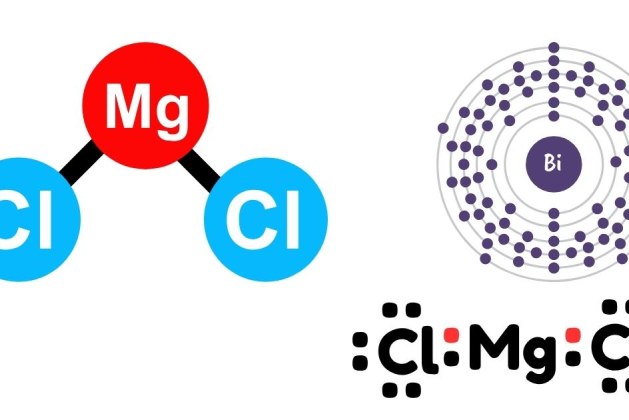
When magnesium chloride reacts with potassium hydroxide, magnesium hydroxide and potassium chloride are formed. The balanced chemical equation is:
In this reaction:
- Magnesium chloride (MgCl₂) provides magnesium ions (Mg²⁺).
- Potassium hydroxide (KOH) provides hydroxide ions (OH⁻).
- Magnesium hydroxide (Mg(OH)₂) precipitates out as an insoluble product.
- Potassium chloride (KCl) remains in solution as a soluble salt.
Key Concepts:
- Stoichiometry:
- The balanced equation shows a 1:2 molar ratio between MgCl₂ and KOH.
- For every mole of MgCl₂, two moles of KOH are required.
- One mole of MgCl₂ produces one mole of Mg(OH)₂ and two moles of KCl.
- Limiting Reactant:
- In reactions involving specific quantities of reactants, the limiting reactant determines the amount of product formed.
- Precipitation Reaction:
- Magnesium hydroxide (Mg(OH)₂) is insoluble in water and forms a solid precipitate, which can be observed during the reaction.
Example Calculation:
Suppose 6 grams of MgCl₂ react with an excess of KOH. How much Mg(OH)₂ will be formed?
Step 1: Calculate the molar mass of each compound:
- MgCl₂:
- Mg(OH)₂:
Step 2: Convert grams of MgCl₂ to moles:
Step 3: Use the stoichiometric ratio to find moles of Mg(OH)₂ formed: Since the ratio of MgCl₂ to Mg(OH)₂ is 1:1:
Step 4: Convert moles of Mg(OH)₂ to grams:
Final Answer:
When 6 grams of MgCl₂ react with excess KOH, approximately 3.67 grams of Mg(OH)₂ will be produced.
This reaction illustrates key principles of stoichiometry and precipitation chemistry. The use of balanced equations and molar relationships allows us to predict the outcome of chemical reactions accurately. Whether in a classroom or a laboratory, understanding these fundamental concepts is essential for anyone exploring the world of chemistry.


Leave a comment