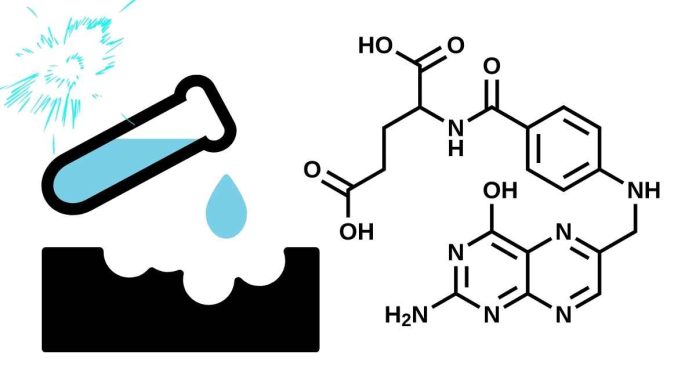
CH₃COONa, or sodium acetate, is not considered an acid but a salt. It forms from the neutralization of acetic acid (CH₃COOH) with sodium hydroxide (NaOH). Sodium acetate is actually a basic salt because its acetate ion (CH₃COO⁻) is the conjugate base of a weak acid, acetic acid. In water, the acetate ion can react with H⁺ from water, slightly increasing the hydroxide ion (OH⁻) concentration, making the solution mildly basic. So, while CH₃COONa itself isn’t acidic, it plays a role in buffering systems and can influence pH levels, depending on its environment.