When we examine chemical compounds, one of the most important questions we ask is whether the compound is ionic or covalent. Understanding this helps us grasp the behavior of the compound in different environments, its physical properties, and how it interacts with other substances. In this blog post, we’ll be taking a closer look at a specific compound: CH₃CH₂CH₂CHCH₂CH₃CH₂COOH, and we’ll explore whether this compound is ionic or covalent.
What is CH₃CH₂CH₂CHCH₂CH₃CH₂COOH?
First, let’s break down the structure of this compound. It is a fatty acid, likely 7-octenoic acid or a related compound. The structure consists of a long carbon chain (made up of carbon atoms bonded to hydrogen atoms) with a carboxyl group (-COOH) at the end. The carboxyl group is a characteristic feature of fatty acids and plays a crucial role in the compound’s properties.
Here’s a simplified breakdown of the formula:
- CH₃CH₂CH₂CHCH₂CH₃CH₂: This is a long hydrocarbon chain composed of carbon and hydrogen atoms. Each carbon atom is bonded to hydrogen atoms, except for the ones that are part of the carboxyl group at the end.
- COOH: This is the carboxyl group (-COOH), which contains a carbon double-bonded to oxygen (C=O) and a hydroxyl group (-OH).
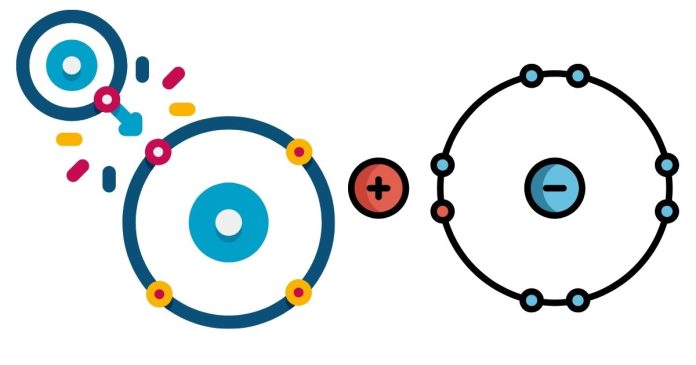
Ionic vs. Covalent Bonds
To determine whether this compound is ionic or covalent, we need to understand the difference between these two types of bonds:
- Ionic Bonds: Ionic bonds are formed when atoms transfer electrons from one to another, resulting in the formation of ions. Typically, ionic bonds occur between metals and nonmetals, where the metal atom gives up electrons and the nonmetal accepts them.
- Covalent Bonds: Covalent bonds occur when two atoms share electrons. These bonds are typically found between nonmetals. Covalent compounds tend to have lower melting and boiling points compared to ionic compounds and don’t conduct electricity in their solid state.
What Type of Bonding Does CH₃CH₂CH₂CHCH₂CH₃CH₂COOH Exhibit?
Let’s analyze the bonds in the compound CH₃CH₂CH₂CHCH₂CH₃CH₂COOH:
- Hydrocarbon Chain (C-H Bonds): In the long carbon chain, the carbon atoms are covalently bonded to hydrogen atoms. Since both carbon and hydrogen are nonmetals, the bond between them is covalent. Specifically, these are nonpolar covalent bonds because the electronegativity difference between carbon and hydrogen is minimal.
- C-C Bonds: The carbon atoms within the chain are bonded to each other through nonpolar covalent bonds. Carbon has a similar electronegativity to other carbon atoms, making the bonds between them equally shared.
- C=O (Carbonyl Group) Bond in COOH: In the carboxyl group (-COOH), the carbon atom is double-bonded to an oxygen atom. This is a polar covalent bond because oxygen is more electronegative than carbon, meaning the shared electrons are pulled closer to the oxygen atom.
- C-OH (Hydroxyl Group) Bond in COOH: The bond between carbon and the hydroxyl group’s oxygen is also polar covalent, as oxygen is more electronegative than carbon.
Is the Compound Ionic or Covalent?
The entire structure of CH₃CH₂CH₂CHCH₂CH₃CH₂COOH is held together by covalent bonds. All of the bonds between the atoms within the molecule (carbon, hydrogen, and oxygen) are covalent. This includes the nonpolar covalent bonds in the hydrocarbon chain and the polar covalent bonds in the carboxyl group.
However, while the bonds within the molecule are covalent, the compound can exhibit some ionic behavior in specific environments. For instance, in aqueous solutions (like in water), the carboxyl group (-COOH) can dissociate to release a proton (H⁺), resulting in the formation of a carboxylate ion (RCOO⁻). This process is reversible, and the dissociation can lead to ionic species in solution. But despite this dissociation, the overall molecule remains covalent in nature.
Conclusion
To sum up, the compound CH₃CH₂CH₂CHCH₂CH₃CH₂COOH is primarily covalent. The bonds between the atoms within the molecule are covalent, as all the atoms involved (carbon, hydrogen, and oxygen) are nonmetals. While the compound may form ions in solution, the molecule itself is not ionic. This illustrates the importance of distinguishing between the type of bonds that hold a molecule together and the behavior it exhibits in solution.
Understanding these bonding characteristics is essential not only for chemistry students but also for anyone interested in how molecules interact in different environments. Whether it’s in biological systems or industrial applications, knowing how compounds like fatty acids behave can help us better understand their roles and applications.