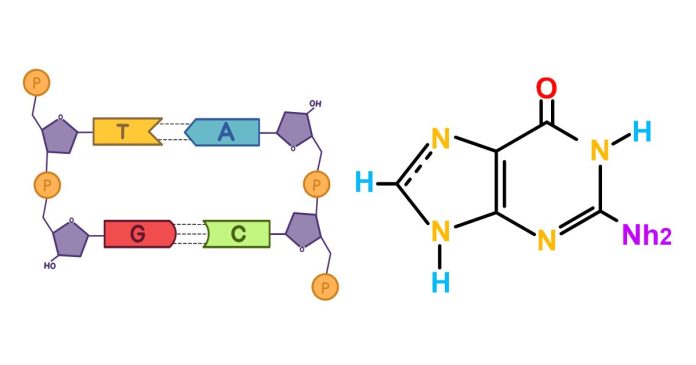
In DNA, Guanine Consistently Pairs Through Hydrogen Bonds With Which Base?
DNA, the molecule that carries the genetic blueprint of life, owes much of its stability to a specific pairing of nitrogenous bases. Among these, guanine (G) plays a crucial role. But with which base does guanine consistently form hydrogen bonds?
The answer lies in the principle of complementary base pairing, a cornerstone of DNA’s double-helix structure. Guanine always pairs with cytosine (C). This bond is not arbitrary but is instead dictated by the formation of three strong hydrogen bonds between the two bases, ensuring a secure and stable connection.
This pairing not only maintains the structural integrity of the DNA molecule but also plays a vital role in accurate DNA replication and information encoding. The triple hydrogen bond between guanine and cytosine makes their pairing more robust than the two hydrogen bonds formed between adenine (A) and thymine (T).
Understanding these pairings offers fascinating insights into how genetic information is stored and transmitted in all living organisms. Guanine and cytosine are a perfect example of nature’s precision at the molecular level, ensuring that life continues seamlessly from one generation to the next.