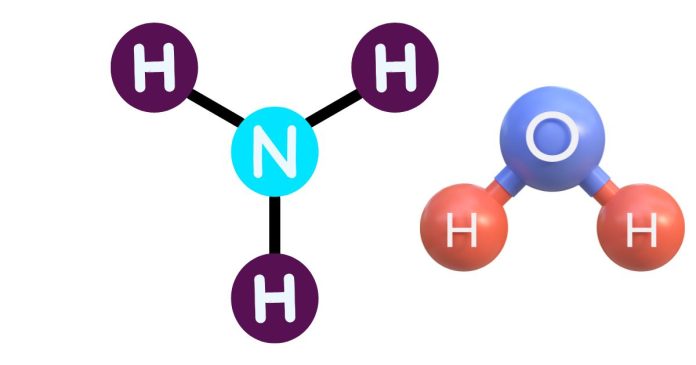
In a chemical formula, subscripts indicate the number of atoms of a particular element in a molecule or compound. They are written as small numbers to the right of the element’s symbol.
For example:
- In H₂O (water), the subscript 2 after the hydrogen (H) indicates that there are two hydrogen atoms in the molecule.
- In CO₂ (carbon dioxide), the subscript 2 after oxygen (O) indicates that there are two oxygen atoms in the molecule.
Key points:
- A subscript of 1 is often implied and not written. For example, in O₂, the “2” tells you that there are two oxygen atoms, but you don’t need to write “O₁” because it is understood.
- Subscripts only apply to the element immediately preceding them and represent the number of atoms of that element in the compound.