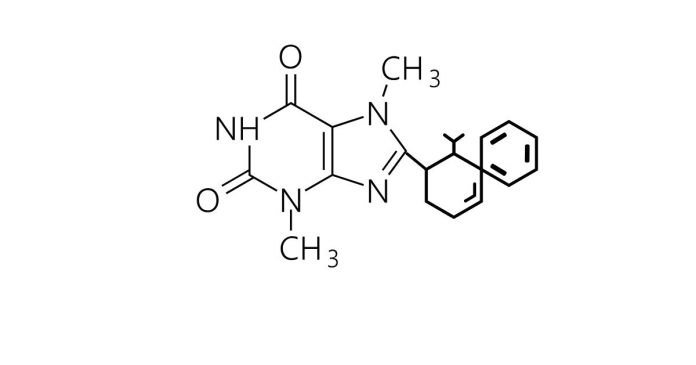
Find the molar mass of the compound:
The molar mass is the mass of one mole of a substance (usually given in grams per mole, g/mol). It is found by adding the atomic masses of all the atoms in the molecule.
Example:
- For water (H₂O), the atomic masses are:
- Hydrogen (H): 1.008 g/mol
- Oxygen (O): 16.00 g/mol
- Molar mass of H₂O = (2 × 1.008) + (1 × 16.00) = 18.016 g/mol
Convert the molar mass to the mass of a single molecule:
To find the mass of one molecule, divide the molar mass by Avogadro’s number (6.022 × 10²³ molecules per mole), which gives the number of molecules in one mole.
Formula:
Mass of one molecule=Avogadro’s number (mol−1)Molar mass (g/mol)
Example Calculation:
For water (H₂O), the molar mass is 18.016 g/mol. To find the mass of one molecule:
Mass of one H₂O molecule=6.022×1023mol−118.016g/mol≈3.00×10−23grams
So, the mass of one water molecule is approximately 3.00 × 10⁻²³ grams.
Summary:
- Find the molar mass of the compound.
- Divide the molar mass by Avogadro’s number (6.022 × 10²³).
- The result gives the mass of one molecule in grams.