Magnesium is an alkaline earth metal located in Group 2 of the periodic table, with the chemical symbol Mg and atomic number 12. One of the key aspects of understanding an element’s behavior is knowing the number of electron shells it has, as this influences its chemical properties, reactivity, and position in the periodic table.
In this article, we will explore how many electron shells magnesium has, and what that means for its properties.
Understanding Electron Shells
Electron shells, also known as energy levels, are regions around an atom’s nucleus where electrons are most likely to be found. Each shell can hold a specific number of electrons:
- First shell: Can hold up to 2 electrons.
- Second shell: Can hold up to 8 electrons.
- Third shell: Can hold up to 18 electrons, and so on.
The arrangement of electrons in these shells follows the aufbau principle, which states that electrons fill the lowest available energy levels first.
Electron Configuration of Magnesium
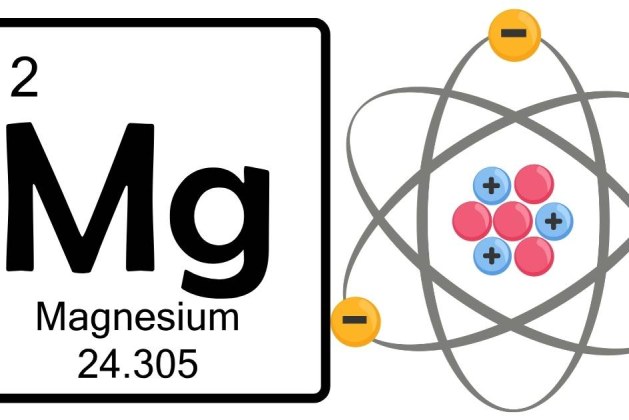
Magnesium, with an atomic number of 12, has a total of 12 electrons. The electron configuration of magnesium describes how these electrons are distributed across the shells.
The electron configuration of magnesium is:
1s² 2s² 2p⁶ 3s².
This means:
- The first shell (1s) contains 2 electrons.
- The second shell (2s and 2p) contains 8 electrons.
- The third shell (3s) contains 2 electrons.
Number of Electron Shells in Magnesium
Based on the electron configuration, magnesium has 3 electron shells:
- First shell (2 electrons)
- Second shell (8 electrons)
- Third shell (2 electrons)
The third shell, although only containing 2 electrons, is still considered a full electron shell, which is why magnesium is classified in the third period (or row) of the periodic table.
Why Does the Number of Electron Shells Matter?
The number of electron shells an element has plays a crucial role in its chemical behavior and reactivity:
- Elements with more electron shells are typically larger in size because the outermost electrons are farther from the nucleus.
- The outermost electrons, found in the valence shell, are most involved in chemical bonding. In magnesium’s case, the 2 electrons in the third shell are its valence electrons.
- Reactivity: Magnesium, having 2 valence electrons, tends to lose them easily to achieve a stable electron configuration (similar to the nearest noble gas, neon), forming a 2+ charge.
Magnesium has 3 electron shells, with its electron configuration being 1s² 2s² 2p⁶ 3s². Understanding the number of electron shells in magnesium helps explain its size, position in the periodic table, and chemical reactivity. The 2 valence electrons in its third shell make magnesium highly reactive, especially in reactions where it can lose these electrons to form Mg²⁺ ions.

Leave a comment