arbon is one of the most versatile elements in the periodic table, and its ability to form covalent bonds is central to its role in chemistry and biology. But how many covalent bonds can each carbon atom form? Let’s explore this fascinating question.
The Basics of Covalent Bonding
Covalent bonds are chemical bonds formed when two atoms share electrons. This sharing allows atoms to achieve a stable electronic configuration, often resembling the noble gases. For carbon, this stability is achieved through its unique electron arrangement.
Carbon’s Electron Configuration
Carbon has an atomic number of 6, which means it has 6 protons and 6 electrons. The electron configuration of carbon is:
1s² 2s² 2p²
This configuration shows that carbon has four valence electrons in its outermost shell (2 in the 2s orbital and 2 in the 2p orbital). To achieve stability, carbon tends to share these four electrons with other atoms, forming covalent bonds.
The Octet Rule and Carbon
The octet rule states that atoms are most stable when they have eight electrons in their outer shell. Since carbon starts with four valence electrons, it needs four more to complete its octet. This is why each carbon atom can form up to four covalent bonds.
Examples of Carbon’s Bonding
Carbon’s ability to form four covalent bonds makes it the backbone of organic chemistry. Here are some examples:
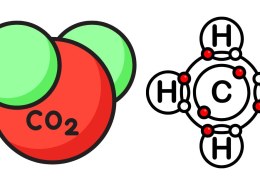
- Methane (CH₄):
- In methane, carbon forms four single covalent bonds with four hydrogen atoms.
- Ethene (C₂H₄):
- In ethene, carbon forms a double bond with another carbon atom and two single bonds with hydrogen atoms.
- Carbon Dioxide (CO₂):
- In carbon dioxide, carbon forms two double bonds with two oxygen atoms.
Why Is Carbon So Versatile?
Carbon’s ability to form four covalent bonds allows it to:
- Create complex molecules with chains, rings, and branches.
- Bond with a variety of elements, including hydrogen, oxygen, nitrogen, and sulfur.
- Form single, double, and triple bonds, providing additional structural diversity.
Each carbon atom can form four covalent bonds, making it uniquely suited to serve as the foundation of life on Earth. This property enables carbon to create the vast array of organic compounds that are essential for biological processes, materials, and countless chemical applications. Understanding carbon’s bonding capabilities is key to appreciating its central role in chemistry and beyond.