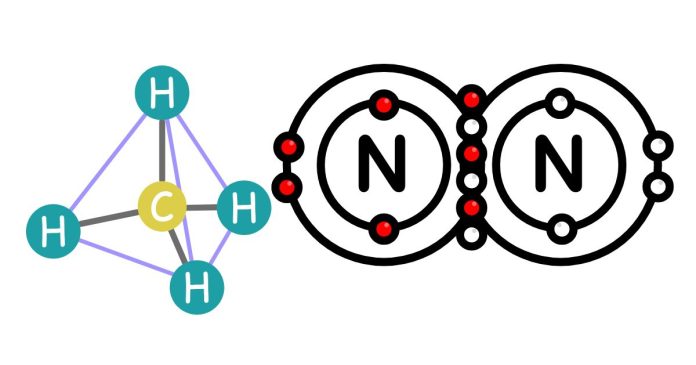
A typical carbon atom can form up to four covalent bonds. This is due to its atomic structure, where carbon has an electron configuration of 1s² 2s² 2p². Its outermost shell contains four electrons, which means it requires four additional electrons to achieve a stable octet, following the octet rule. To meet this requirement, carbon atoms bond with other atoms by sharing electrons. These bonds can be single, double, or triple covalent bonds, depending on how many pairs of electrons are shared. Carbon’s ability to form four bonds is a key reason why it is the backbone of organic chemistry, allowing it to form a wide variety of molecules like hydrocarbons, carbohydrates, proteins, and DNA.
How Many Covalent Bonds Can a Typical Carbon Atom Form?
RELATED ARTICLES
0 Comments
Oldest