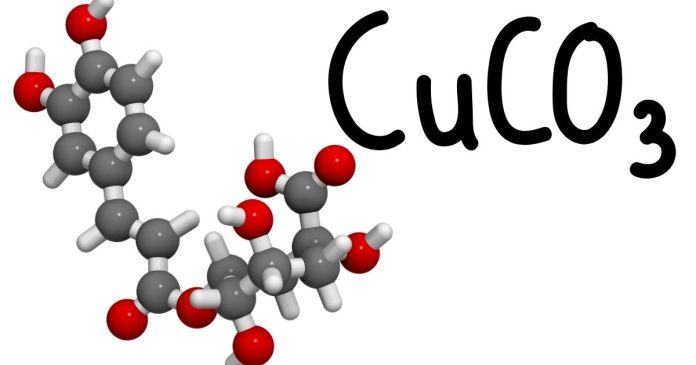
The formula for copper(II) carbonate is CuCO₃. To count the atoms, we need to look at the elements involved in the compound and how many atoms of each element are present.
- Copper (Cu): The “Cu” represents copper. There is only 1 copper atom in the formula.
- Carbon (C): The “C” represents carbon. There is 1 carbon atom in the formula.
- Oxygen (O): The “O₃” means that there are 3 oxygen atoms in the formula.
Now, to calculate the total number of atoms in CuCO₃:
- 1 copper atom (Cu)
- 1 carbon atom (C)
- 3 oxygen atoms (O)
Adding these together gives the total number of atoms:
- 1 (Cu) + 1 (C) + 3 (O) = 5 atoms
So, in one molecule of CuCO₃, there are 5 atoms in total.