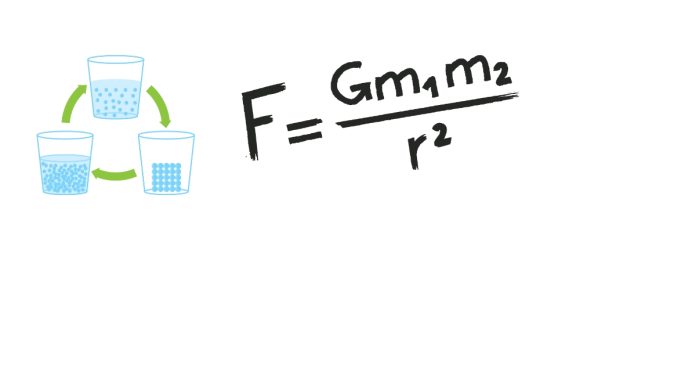How do you balance the Equation NaCl + F₂ → NaF + Cl₂?
To balance the equation NaCl + F₂ → NaF + Cl₂, you need to balance the number of sodium (Na), chlorine (Cl), and fluorine (F) atoms.
The balanced equation is:
\[ 2NaCl + F_2 \rightarrow 2NaF + Cl_2 \]