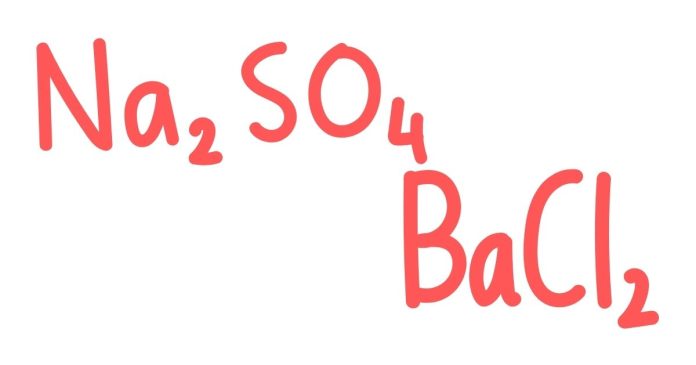
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is followed. This means that the number of atoms of each element must be the same on both sides of the equation. Let’s break down the process of balancing the reaction between barium chloride (BaCl₂) and sodium sulfate (Na₂SO₄).
Step 1: Write the Unbalanced Equation
The unbalanced chemical equation for the reaction is:
BaCl₂ + Na₂SO₄ → BaSO₄ + NaCl
This represents the double displacement reaction where barium sulfate (BaSO₄) and sodium chloride (NaCl) are formed.
Step 2: Identify the Number of Atoms of Each Element
Initially, count the number of atoms of each element on both sides of the equation:
Reactants:
- Ba: 1
- Cl: 2
- Na: 2
- S: 1
- O: 4
Products:
- Ba: 1
- Cl: 1
- Na: 1
- S: 1
- O: 4
Step 3: Balance the Atoms
The goal is to make the number of atoms of each element equal on both sides. Let’s address the imbalance:
- Chlorine (Cl) and Sodium (Na): There are 2 chlorine atoms in BaCl₂ on the reactants’ side but only 1 chlorine atom in NaCl on the products’ side. Similarly, there are 2 sodium atoms in Na₂SO₄ but only 1 sodium atom in NaCl. To balance, place a coefficient of 2 in front of NaCl:
BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl
2. Check Other Elements:
-
- Ba: 1 atom on both sides.
- S: 1 atom on both sides.
- O: 4 atoms on both sides.
At this point, all elements are balanced.
Step 4: Verify the Balancing
Recount the atoms to ensure the equation is balanced:
Reactants:
- Ba: 1
- Cl: 2
- Na: 2
- S: 1
- O: 4
Products:
- Ba: 1
- Cl: 2 (2 NaCl molecules)
- Na: 2 (2 NaCl molecules)
- S: 1
- O: 4
Since the number of atoms of each element is the same on both sides, the equation is balanced.
Final Balanced Equation
The balanced chemical equation for the reaction is:
BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl
Key Takeaways
- Always start by writing the unbalanced equation.
- Count the atoms of each element on both sides.
- Use coefficients to balance the number of atoms for each element.
- Double-check your work to ensure the equation obeys the law of conservation of mass.
Balancing equations like this one is a crucial step in understanding chemical reactions and their stoichiometry. Practice with other reactions to become proficient!