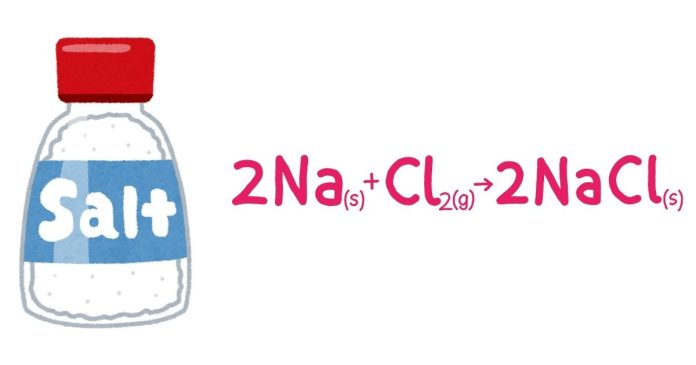How a Salt is Obtained in a Reaction
Salts are formed through a variety of chemical reactions, but the most common and well-known method is a process called neutralization. This occurs when an acid reacts with a base, leading to the formation of a salt and water. Salts are ionic compounds that play an essential role in many areas of chemistry, biology, and industry.
The Neutralization Reaction:
The most basic way to obtain a salt is through the neutralization of an acid and a base. Here’s how it works:
Acid + Base → Salt + Water
- An acid is a substance that donates hydrogen ions (H⁺) when dissolved in water. For example, hydrochloric acid (HCl).
- A base is a substance that accepts hydrogen ions or donates hydroxide ions (OH⁻). A common base is sodium hydroxide (NaOH).
When an acid and a base are mixed, they interact to produce a salt and water. The hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions, the positive ions (cations) from the base and the negative ions (anions) from the acid, combine to form the salt.
For example:
- Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H₂O).
HCl + NaOH → NaCl + H₂O
Types of Salts:
The type of salt formed depends on the acid and base used in the reaction. For example:
- Sodium chloride (NaCl): Produced from the neutralization of hydrochloric acid (HCl) with sodium hydroxide (NaOH).
- Copper(II) sulfate (CuSO₄): Formed when sulfuric acid (H₂SO₄) reacts with copper(II) oxide (CuO), a base.
- Calcium carbonate (CaCO₃): Formed when calcium hydroxide (Ca(OH)₂) reacts with carbonic acid (H₂CO₃).
Other Ways to Obtain Salts:
While neutralization is the most common method, salts can also be obtained through other reactions such as:
- Displacement Reactions: Where a more reactive metal displaces a less reactive one from its salt solution.
- Direct Combination: When elements like an alkali metal and a halogen react directly to form a salt.
Applications of Salts:
Salts are incredibly versatile and have numerous uses:
- In industry, salts are used in manufacturing, water treatment, and as preservatives.
- In biology, salts help maintain proper cell function and fluid balance.
- In everyday life, table salt (NaCl) is a household staple for cooking and preservation.
In summary, a salt is typically obtained through a neutralization reaction between an acid and a base, producing water and an ionic compound known as a salt. This process is foundational in chemistry and has widespread applications across various fields. Whether in laboratories, industries, or the kitchen, salts play a vital role in both science and daily life.