When discussing chemistry, the concept of miscibility often arises, particularly when exploring how different liquids interact with one another. Miscibility refers to the ability of two substances to mix in all proportions without separating into two phases. One common question is whether water (H2O) and methanol (CH3OH) are miscible. The answer is a resounding yes, and here’s why.
Understanding Miscibility
To understand why H2O and CH3OH are miscible, we need to dive into their molecular structures and the nature of their interactions. Miscibility is primarily governed by intermolecular forces—the forces that occur between molecules. These forces determine whether two substances can mix evenly or separate into distinct layers.
The Chemistry of Water (H2O)
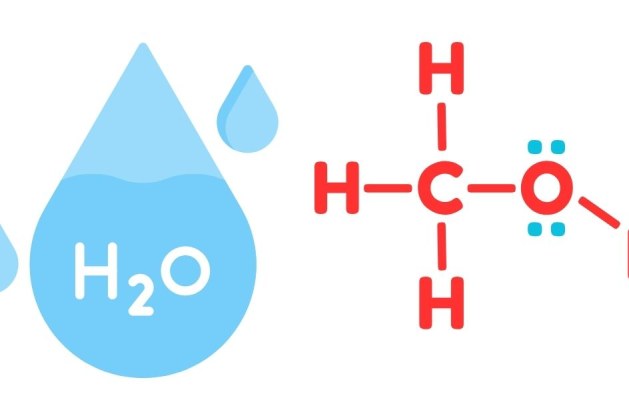
Water is a polar molecule with a bent structure due to its two hydrogen atoms and one oxygen atom. The oxygen atom is highly electronegative, meaning it pulls electrons closer to itself, creating a partial negative charge at the oxygen end and a partial positive charge at the hydrogen ends. This polarity allows water molecules to form hydrogen bonds with each other.
The Chemistry of Methanol (CH3OH)
Methanol, also known as methyl alcohol, consists of a hydroxyl (-OH) group attached to a single carbon atom. Like water, methanol is a polar molecule because of its hydroxyl group, which can participate in hydrogen bonding. Additionally, the carbon-hydrogen part of methanol provides some nonpolar characteristics, but the molecule remains predominantly polar.
Why Are H2O and CH3OH Miscible?
- Hydrogen Bonding: Both water and methanol can form hydrogen bonds due to the presence of -OH groups in their structures. When mixed, water molecules and methanol molecules interact through these hydrogen bonds, creating a homogenous mixture.
- Polarity Compatibility: The polar nature of both H2O and CH3OH ensures that their molecules are attracted to one another. Like dissolves like, a common principle in chemistry, applies here.
- Size and Structure: Methanol’s small size and relatively simple structure make it highly compatible with water molecules. There is no significant steric hindrance that might prevent their interaction.
Real-World Applications
The miscibility of water and methanol has numerous practical implications, including:
- Fuel Blends: Methanol is often used in fuel mixtures with water for combustion engines.
- Solvent Systems: The water-methanol combination is commonly used in laboratories and industries as a solvent for various chemical reactions.
- Antifreeze Solutions: Methanol’s miscibility with water makes it an excellent antifreeze agent in colder climates.
Water (H2O) and methanol (CH3OH) are fully miscible due to their ability to form hydrogen bonds and their shared polarity. This compatibility allows them to mix uniformly in any proportion, making their miscibility a textbook example of how molecular structure influences chemical behavior.
Understanding the miscibility of substances like H2O and CH3OH not only deepens our grasp of fundamental chemistry but also highlights the practical applications of such interactions in everyday life and industry.


Leave a comment